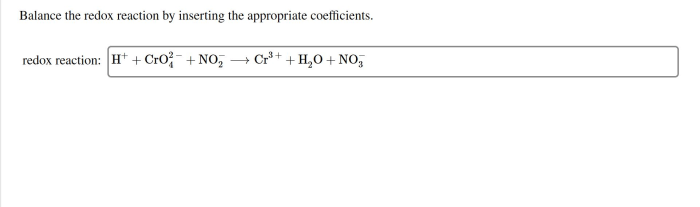
Rank the radicals in order of decreasing stability – In the realm of chemistry, the stability of radicals plays a pivotal role. This discourse delves into the concept of radical stability, exploring its significance and the factors that govern it. By examining practical applications and theoretical approaches, we uncover the intricacies of radical stability, shedding light on its profound influence in various scientific disciplines.
Radical Stability Ranking
Radical stability refers to the relative tendency of a radical to undergo reactions that lead to its loss. Radicals are atoms or molecules with unpaired electrons, and they are highly reactive due to the presence of the unpaired electron.
The stability of a radical is determined by several factors, including resonance, hyperconjugation, electronegativity, and inductive effects. Resonance and hyperconjugation can stabilize radicals by delocalizing the unpaired electron, while electronegativity and inductive effects can destabilize radicals by withdrawing electron density from the radical center.
Table of Radicals in Order of Decreasing Stability
| Radical | Stability |
|---|---|
| Methyl | Least stable |
| Ethyl | More stable than methyl |
| Isopropyl | More stable than ethyl |
| tert-Butyl | Most stable |
Factors Affecting Radical Stability
Resonance
Resonance is a major factor that can stabilize radicals. Resonance occurs when there are multiple Lewis structures for a molecule or radical, and the unpaired electron can be delocalized over several atoms. This delocalization reduces the energy of the radical and makes it more stable.
Hyperconjugation
Hyperconjugation is another factor that can stabilize radicals. Hyperconjugation occurs when there is overlap between a sigma bond and a p orbital on an adjacent atom. This overlap can delocalize the unpaired electron and reduce the energy of the radical.
Electronegativity
Electronegativity is a measure of the ability of an atom to attract electrons. Electronegative atoms can destabilize radicals by withdrawing electron density from the radical center. This withdrawal of electron density makes the radical more reactive and less stable.
Inductive Effects
Inductive effects are another factor that can destabilize radicals. Inductive effects occur when there is a difference in electronegativity between two atoms that are bonded to each other. The more electronegative atom will withdraw electron density from the less electronegative atom, which can destabilize the radical.
Applications of Radical Stability

Organic Chemistry, Rank the radicals in order of decreasing stability
Radical stability is an important concept in organic chemistry, as radicals are involved in a wide variety of reactions. Radicals can be generated by a variety of methods, including homolytic bond cleavage, heterolytic bond cleavage, and electron transfer. Radicals can then react with a variety of other molecules, including alkenes, alkynes, and arenes.
Polymer Chemistry
Radical stability is also an important concept in polymer chemistry, as radicals are involved in the polymerization of many different types of polymers. Radicals can be generated by a variety of methods, including the use of initiators, and they can then react with monomers to form polymers.
The stability of the radicals involved in the polymerization process can affect the properties of the resulting polymer.
Biochemistry
Radical stability is also an important concept in biochemistry, as radicals are involved in a variety of biological processes. Radicals can be generated by a variety of methods, including the metabolism of oxygen, and they can then react with a variety of biological molecules, including DNA, proteins, and lipids.
The stability of the radicals involved in these processes can affect the health and function of cells.
Methods for Measuring Radical Stability
Electron Spin Resonance (ESR) Spectroscopy
ESR spectroscopy is a technique that can be used to measure the concentration of radicals in a sample. ESR spectroscopy works by detecting the unpaired electrons in radicals, and it can be used to measure the concentration of radicals in a variety of different samples.
Chemiluminescence
Chemiluminescence is a technique that can be used to measure the rate of radical reactions. Chemiluminescence occurs when radicals react with each other to produce light, and the intensity of the light can be used to measure the rate of the reaction.
Trapping Experiments
Trapping experiments are another technique that can be used to measure the stability of radicals. Trapping experiments involve reacting radicals with a trapping agent, which is a molecule that reacts with radicals to form a stable product. The amount of product that is formed can then be used to measure the concentration of radicals in the sample.
Theoretical Approaches to Radical Stability: Rank The Radicals In Order Of Decreasing Stability

Density Functional Theory (DFT)
DFT is a computational method that can be used to predict the stability of radicals. DFT uses a variety of mathematical techniques to calculate the energy of a molecule or radical, and it can be used to predict the stability of radicals in a variety of different environments.
Molecular Orbital Theory
Molecular orbital theory is another computational method that can be used to predict the stability of radicals. Molecular orbital theory uses a variety of mathematical techniques to calculate the wavefunction of a molecule or radical, and it can be used to predict the stability of radicals in a variety of different environments.
Valence Bond Theory
Valence bond theory is a computational method that can be used to predict the stability of radicals. Valence bond theory uses a variety of mathematical techniques to calculate the energy of a molecule or radical, and it can be used to predict the stability of radicals in a variety of different environments.
FAQ Insights
What is radical stability?
Radical stability refers to the relative resistance of a radical to undergoing chemical reactions that lead to its decomposition.
How is radical stability measured?
Experimental techniques such as electron spin resonance (ESR) spectroscopy and chemiluminescence are used to measure radical stability.
What factors affect radical stability?
Factors that influence radical stability include resonance, hyperconjugation, electronegativity, and inductive effects.