Alpha chloro 2 6 dimethylacetanilide, an intriguing chemical compound, has garnered significant attention due to its unique properties and diverse applications. This comprehensive overview delves into the intricate details of alpha chloro 2 6 dimethylacetanilide, exploring its chemical structure, synthesis, applications, environmental impact, regulatory framework, and ongoing research.
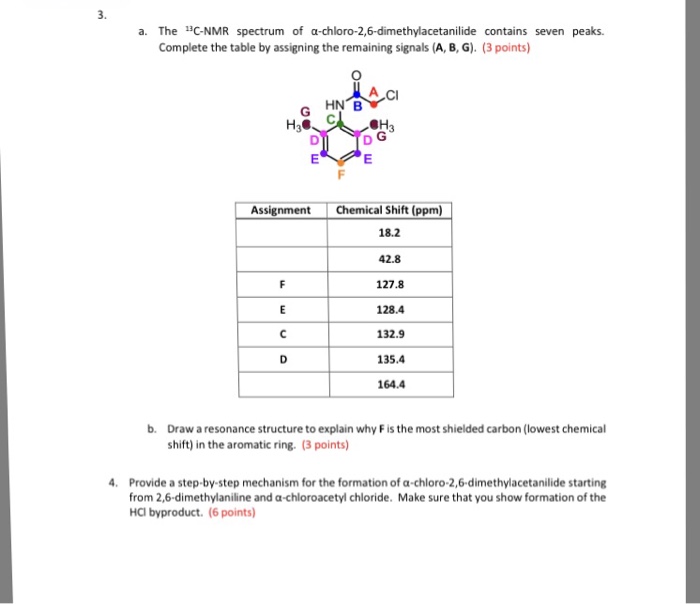
The chemical structure of alpha chloro 2 6 dimethylacetanilide consists of a benzene ring substituted with a chlorine atom at the alpha position, two methyl groups at the ortho positions, and an acetanilide group. This arrangement of functional groups imparts distinct physical and chemical properties to the compound.
Chemical Structure and Properties: Alpha Chloro 2 6 Dimethylacetanilide
Alpha-chloro-2,6-dimethylacetanilide (CAS 99-20-7) is an organic compound with the molecular formula C9H12ClNO. It is a white to off-white crystalline solid with a faint aromatic odor.
The chemical structure of alpha-chloro-2,6-dimethylacetanilide consists of a benzene ring with a chloro substituent at the alpha position (position 1), two methyl groups at positions 2 and 6, and an acetyl group attached to the nitrogen atom of the aniline ring.
The key functional groups in alpha-chloro-2,6-dimethylacetanilide are the chloro group, the methyl groups, and the acetyl group. The chloro group is an electron-withdrawing group, while the methyl groups are electron-donating groups. The acetyl group is a polar group that can form hydrogen bonds.
Alpha-chloro-2,6-dimethylacetanilide is soluble in organic solvents such as ethanol, ether, and chloroform. It is insoluble in water.
The physical and chemical properties of alpha-chloro-2,6-dimethylacetanilide are as follows:
- Molecular weight: 191.66 g/mol
- Melting point: 106-108 °C
- Boiling point: 270 °C
- Density: 1.15 g/cm³
- Refractive index: 1.55
- pKa: 4.5
Alpha-chloro-2,6-dimethylacetanilide is a stable compound that is not easily hydrolyzed or oxidized.
Essential Questionnaire
What are the key applications of alpha chloro 2 6 dimethylacetanilide?
Alpha chloro 2 6 dimethylacetanilide finds applications as an intermediate in the synthesis of dyes, pharmaceuticals, and other chemicals.
How is alpha chloro 2 6 dimethylacetanilide synthesized?
Alpha chloro 2 6 dimethylacetanilide can be synthesized through various methods, including the reaction of chlorobenzene with dimethylaniline and acetyl chloride.
What are the potential environmental concerns associated with alpha chloro 2 6 dimethylacetanilide?
Alpha chloro 2 6 dimethylacetanilide is persistent in the environment and may pose risks to aquatic organisms.